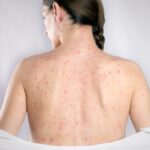
Leprosy, also known as Hansen’s disease, is a persistent granulomatous infection affecting the skin, peripheral nerves, and upper respiratory tract mucosa. This condition is instigated by Mycobacterium leprae, an acid-fast bacillus. Characterized by its ancient roots and unfortunate neglect, leprosy inflicts tissue damage and demyelinating lesions within peripheral nerves. Once prevalent in Europe and Asia, leprosy now prevails in 105 countries, primarily within developing nations and regions of poverty, where warm temperate tropical climates foster its spread.
The human immune response to M. leprae manifests in diverse clinical presentations. From the tuberculoid form, marked by robust production of Th1-type cytokines, to the lepromatous variant, characterized by elevated levels of Th2-type cytokines and a suboptimal proinflammatory reaction. Diagnosing leprosy involves a multifaceted approach encompassing clinical observation, histopathological analysis of biopsies obtained from the active edge of lesions, bacteriological and molecular methods, as well as the lepromine skin test.
This review aims to commemorate the 150th anniversary of Dr. Gerhard-Henrik Armauer Hansen’s groundbreaking discovery of M. leprae, shedding light on the current understanding of its pathogenesis and microbiological intricacies.
Historical Background
For centuries, individuals afflicted with leprosy have faced ostracization, stigmatization, and judgment from society. Particularly in Western cultures, leprosy has been attributed as a divine punishment or curse. However, Dr. Gerhard-Henrik Armauer Hansen, a Norwegian scientist, challenged the theories of hereditary or divine curse regarding the etiology of the disease. Instead, he asserted that leprosy was an infectious condition and went on to discover the causative agent, M. leprae. Significant milestones in the history of leprosy include:
- Discovery of the bacillus by Dr. Armauer Hansen in 1873 in Norway.
- Introduction of sulfone drugs for treatment by Dr. Guy Henry Faget in 1941 in Carville.
- Production of the bacillus on the footpads of mice by Dr. Charles Shepard in 1959 at the CDC, USA.
- Inoculation of leprosy bacilli into nine-banded armadillos by Dr. Waldemar Kirchheimer and Dr. Eleanor Storrs, demonstrating the susceptibility of this animal to Hansen’s disease in 1968 at the South Gulf Research Institute, Carville.
Dr. Gerhard Henrik Armauer Hansen studied at the Christiania University Medical Faculty and had the opportunity to work with Dr. Daniel C. Danielssen and Dr. Carl W. Boeck, who were leading authorities on leprosy research in Europe. During that time, many scientists, including Dr. Danielssen, advocated for the hereditary transmission theory of the disease. Given the long incubation period post-contact, the concept of the disease’s contagiousness was not yet widely acknowledged.
In the 1850s, approximately 0.2% of the population in Norway and 2.5% in Bergen were estimated to have leprosy. Dr. Hansen, along with Dr. Danielssen, visited leprosy patients across Norway, collecting samples from their lesions to examine and prove the contagious nature of the disease. In an era where the concept of transmission was not well understood, contemplating and sharing the theory of contagion was indeed a bold move. Despite facing significant criticism from his mentor Dr. Danielssen and other scientists for his theory of disease transmission via contagion, Hansen persisted in his research to substantiate his theory.
In his initial publication in 1869, Dr. Hansen described pathological changes in leprous tissue. However, expecting Hansen to effectively identify the leprosy bacillus with inadequate equipment and extremely primitive staining techniques would have been unfair. In 1870, during his advanced training in Vienna in staining and histopathology, he refined his technique. Finally, in 1873, at the young age of 32, his research on identifying the infectious agent in leprous patient material successfully concluded, and he published his historic study.
Identifying rod-shaped bodies resembling bacteria in tissues taken from leprosy nodules, Dr. Hansen asserted that these bacteria were the causative agents of leprosy and refuted the notion of leprosy as a divine punishment or the consequence of lepers’ sins. In fact, Dr. Hansen was the first researcher to propose that microorganisms could cause human diseases. Despite facing significant criticism from colleagues who found his explanation and ambitious proposal amusing, believing it to be a product of Hansen’s imagination, he never wavered from his thesis that microorganisms were responsible for leprosy.
Many scientists rejected Hansen’s theory as it did not comply with Koch’s postulates. Desperate to fulfill Koch’s assumptions, Hansen resorted to unethical and reckless experimentation on a woman’s eye without her consent, inoculating material from a leper. His bold and unethical attempt to conduct experiments on humans led him into a medico-legal quagmire. In 1880, Hansen was found guilty in court for violating medical ethics by conducting experiments on humans and was dismissed from Bergen Hospital.
Albert Neisser, a German bacteriologist and a student of Robert Koch, traveled to Norway in 1879 to study the disease and obtained preparations from leprosy nodules prepared by Dr. Hansen. Upon his return to Germany, Neisser claimed credit for his scientific finding in 1880 without mentioning Dr. Hansen, asserting that the honor of discovering the organism causing leprosy belonged to him. News of Neisser’s plagiarism reached Norway, and the scientific community, especially Dr. Danielssen’s student Hansen, defended Hansen’s rights. Eventually, at a Leprosy Congress in Berlin, Hansen was officially declared the discoverer, and in his honor, leprosy was termed “Hansen’s Disease,” and the leprosy-causing bacillus was named “Hansen’s Bacillus.”
The enactment of a law mandating the cautious isolation of lepers from the unaffected segments of society in 1885, following Hansen’s tireless efforts, resulted in a rapid and steady decline in leprosy burden in Norway.
Epidemiology
Leprosy, one of the oldest diseases known to humankind, is believed to have spread across continents from India, China, and Egypt through colonization, trade, military invasions, slavery, and mass migrations.
Designated as one of the six major threats in developing countries by the World Health Organization (WHO), leprosy, although treatable, often leads to severe, lifelong disabilities and deformities due to delayed or misdiagnosis. The primary obstacles to leprosy eradication include current limitations in understanding the disease’s diagnosis and transmission pathways and the increasing incidence of multidrug-resistant cases.
While the incidence of the disease significantly decreased with the initiation of multidrug therapy in the 1980s, leprosy remains endemic in tropical countries, particularly in underdeveloped or developing nations. Although the frequency of the disease has decreased considerably in many regions, it remains prevalent in Southeast Asia, the Americas, Africa, the Pacific, and the Western Mediterranean, with reports of it being endemic in 105 countries.
Currently, more than 200,000 new cases of leprosy are reported annually in over 120 countries worldwide. Global efforts to eliminate leprosy as a public health concern have been successful in many countries, defined as less than one prevalence per 10,000 population, between 2000 and 2010. However, Brazil, India, and Indonesia reported more than 10,000 new cases each in 2019, while 13 other countries reported between 1,000 and 10,000 new cases each (Bangladesh, Democratic Republic of the Congo, Ethiopia, Madagascar, Mozambique, Myanmar, Nepal, Nigeria, the Philippines, Somalia, South Sudan, Sri Lanka, and the United Republic of Tanzania). Additionally, less than 1,000 new cases were reported in 99 countries, with no new cases reported in 45 countries.
Bacteriology
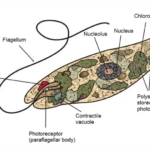
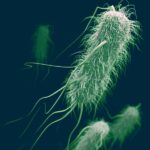
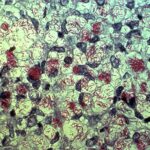
Mycobacterium leprae belongs to the class Schizomycetes, the order Actinomycetales, the family Mycobacteriaceae, and the genus Mycobacterium. Under microscopic examination, they appear as immobile rod-shaped bacilli, ranging from 1.5 to 8 microns in length and 0.2 to 0.3 microns in width. Due to the high lipid content of their cell wall, they exhibit acid-fast bacilli (AFB) characteristics when stained using the Ziehl-Neelsen (ZN) staining method. Unlike other mycobacteria, M. leprae forms parallel arrays known as “globi,” resembling bundles of cigars. Under Gram staining, M. leprae may appear as ghost-like Gram-negative images or as Gram-positive bead-like bacilli.
The fine structure of Mycobacterium leprae is similar to other mycobacteria. Electron microscopy has shown that the bacterium has structures including cytoplasm, plasma membrane, cell wall, and capsule. The cytoplasm contains structures shared with Gram-positive microorganisms. The plasma membrane consists of a permeable bilayer lipid membrane containing proteins associated with surface antigens. Attached to the plasma membrane, the cell wall, similar to other mycobacteria, is composed of branched-chain polisaccharides bound to peptidoglycan, arabinogalactan supporting mycolic acids, and lipoarabinomannan. The outermost layer, the capsule, primarily consists of lipid structures, particularly phenolic glycolipid (PGL-1) bound to deficiatoseroldimycocerosate and phenolic trisaccharides. This trisaccharide serves as the specific antigen of M. leprae.
The genome of Mycobacterium leprae was sequenced in 2001 by Cole et al., revealing a circular genome with a molecular weight of approximately 2.2 x 109 daltons and a G+C content of 57.8%. Mycobacterium leprae is microaerophilic and ideally grows at temperatures ranging from 30 to 35°C. In infected tissues, hundreds of bacilli gather to form clusters known as globi. M. leprae has not yet been cultured in vitro, and its generation time is very long (14 days). While the average incubation period is reported to be 2-5 years, it can extend up to 10-20 years.
In 1960, Charles C. Shepard conducted inoculation of M. leprae on the footpads of Swiss albino mice, enabling the culture of experimental animals, examination and development of antibiotic treatments, and determination of antibiotic resistance. Kirchheimer and Storrs in 1971 described nine-banded armadillos with body temperatures between 30°C and 35°C as an excellent animal model sensitive to M. leprae. This animal model facilitated the production of large quantities of bacilli and served as the basis for numerous antigenic and genetic studies conducted in the 1980s and 1990s, eventually leading to the sequencing of the M. leprae genome in 2001.
In 2008, Han et al. identified a new species known as M. lepromatosis in two patients presenting with diffuse lepromatous leprosy. The whole genome sequencing results of this new mycobacterium species (M. lepromatosis) demonstrated its close phylogenetic resemblance to M. leprae, suggesting they have a common ancestor.
Transmission Routes of Mycobacterium leprae
Human, African green monkeys, and nine-banded armadillos in Louisiana have been reported as the main reservoirs of infection. The transmission of leprosy requires prolonged close contact between susceptible and genetically predisposed individuals and untreated multibacillary (MB) leprosy patients. Transmission occurs through the inhalation of bacilli present in the respiratory secretions of infected individuals by susceptible individuals. The nasal mucosa serves as the primary entry or exit route for M. leprae. While there is conclusive evidence of transmission via inhalation of infected aerosols, the exact transmission routes of M. leprae are still not fully understood, indicating that they are far more complex than previously thought. Nine-banded armadillos (Dasypus novemcinctus) endemic in the Americas are known to be another natural host and reservoir of M. leprae. Given this, it has been suggested that both anthroponotic and zoonotic transmission are possible.
Studies have shown that ground squirrels (Ictidomystridecem lineatus) are susceptible to M. leprae infection. Recent research has demonstrated that red squirrels (Sciurus vulgaris) in England and Scotland are infected with M. lepromatosis or M. leprae. Meredith et al. reported dermatitis in six red squirrels from various locations in Scotland. All animals exhibited bilateral variably alopecic areas and cutaneous swelling in the nasal region, lips, eyelids, ear pinnae, and distal parts of all limbs. Histological examination revealed numerous acid-fast bacilli (AFB) along with granulomatous dermatitis and epithelioid macrophages in three squirrels. Sequencing of the amplicons obtained from PCR analysis of the hsp65 gene region revealed a 99% sequence homology with M. lepromatosis.
Research on environmental reservoirs has also been conducted. Lavania et al. collected 18 soil samples from 15 villages near the National Leprosy Eradication Institute (JALMA) in India. Six of these samples tested positive for M. leprae by PCR. Mohanty et al. collected soil and water samples from leprosy-endemic areas in India and control samples from areas where leprosy was not thought to be present. While 25.4% of soil samples and 24.2% of water samples from leprosy-endemic regions tested positive for M. leprae 16S rRNA, all control samples tested negative.
There is a known connection between intracellular microorganisms and free-living amoebae. Wheat et al. found that M. leprae can survive for up to 35 days in Acanthamoeba echinulata, Acanthamoeba castellanii, Acanthamoeba polyphagave, and Hartmannella vermiformis, and up to eight months in amoebic cysts.
Risk
Living in endemic areas, the clinical classification of the index patient, age, physical proximity, and genetic proximity are the main risk factors. Blood relation with the index leprosy patient, cohabitation, and the clinical classification of the leprosy patient are the most important risk parameters for transmission. Contacts with multibacillary (MB) leprosy patients carry a higher risk of transmission compared to contacts with paucibacillary (PB) leprosy patients, and an increased number of lesions also increases the risk of transmission. Elderly individuals are at greater risk, with an increased risk between ages 5-15 and 15-20, followed by a decrease in risk between ages 20-29, and a gradual increase in risk again after age 30. Some studies have indicated a higher prevalence of leprosy in women, and it has been theorized that the immunological effects of pregnancy in young adult women may theoretically lead to higher leprosy incidence in this age group. Gender differences were not observed in studies where pregnant women were excluded from the analysis.
In the general population and among contacts, Bacille Calmette-Guerin (BCG) vaccination has been shown in case-control studies to provide protection against M. leprae.
Treatment
The World Health Organization (WHO) recommends a multidrug therapy regimen for preventing resistance. Treatment schemes for paucibacillary (PB) and multibacillary (MB) forms, as well as for adults and children, have been established based on bacillary count criteria. Dapsone’s most significant advantage is its rapid reduction of infectivity in infected individuals and its ability to decrease relapse rates.
As a second-line treatment, minocycline, ofloxacin, and clarithromycin are also used. Severe nerve damage and musculoskeletal deformities in leprosy patients significantly affect individuals’ schooling discrimination and social life. Therefore, early diagnosis and treatment, especially in children, can reduce transmission and sequelae of the disease. Difficulties in children taking tablets and capsules, as well as the unavailability of oral solution forms at all times, affect treatment adherence.
Prevention
Prophylactic immunization with vaccines such as BCGLepVax and Mycobacterium indicus pranii (MIP), which have proven efficacy, can be administered before or after exposure for prophylactic purposes. However, currently, the only vaccine administered for leprosy prevention is BCG. In a study conducted in India with multibacillary leprosy cases (up to 12 years old) presenting to a tertiary hospital, the proportion of the unvaccinated group was found to be significantly higher than the BCG-vaccinated group (p=0.0352). This study highlights the role of BCG vaccination in enhancing cell-mediated immunity. The protective efficacy of BCG vaccine against leprosy is estimated to be between 20-90%.
For contacts of leprosy patients, both adults and children (≥2 years old), single-dose rifampicin is recommended as prophylactic treatment. It has been reported that single-dose rifampicin, when administered alongside BCG vaccination during childhood, demonstrates an 80% cumulative protective efficacy.