Oncolytic viruses (OVs) are naturally occurring or genetically modified viruses used in cancer therapy. These viruses target tumor cells, replicating within them and breaking down their cell membranes, ultimately leading to their destruction. OVs can also deliver eukaryotic transgenes to tumor cells, trigger anti-tumor immune responses, and support immunogenic cell death, further contributing to the demise of tumor cells.
Through genetic manipulations, the sensitivity of OVs to tumor cells can be enhanced, and mechanisms targeting healthy cells can be weakened, thus reducing their toxicity. Most early-phase OV therapy studies have shown that OVs induce tolerable levels of toxicity in patients. So far, four different OV therapies have been approved by health institutions in various countries for different types of cancer.
The History of OVs and Recombinant OVs
The concept of viruses selectively targeting cancer cells and being utilized as a treatment for cancer began to take shape in 1912 when a leukemia patient experienced necrosis and remission in her tumor following a viral infection. Between the 1950s and 1980s, OVs were explored for developing cancer therapies, but many of these endeavors proved weak and inadequate in clinical stages. However, advancements in the field of genetics nowadays enable viruses to be programmed to specifically target cancer cells while avoiding replication in healthy cells through genetic manipulations like gene silencing, which involves shutting down certain genes.
Studies revealing cancer mechanisms have shown that OVs are more effective when used in combination with drugs such as other immune checkpoint inhibitors. These strategies, aimed at enhancing the specificity and sensitivity of OVs, have led to the approval of recombinant virus-based drugs like T-VEC.
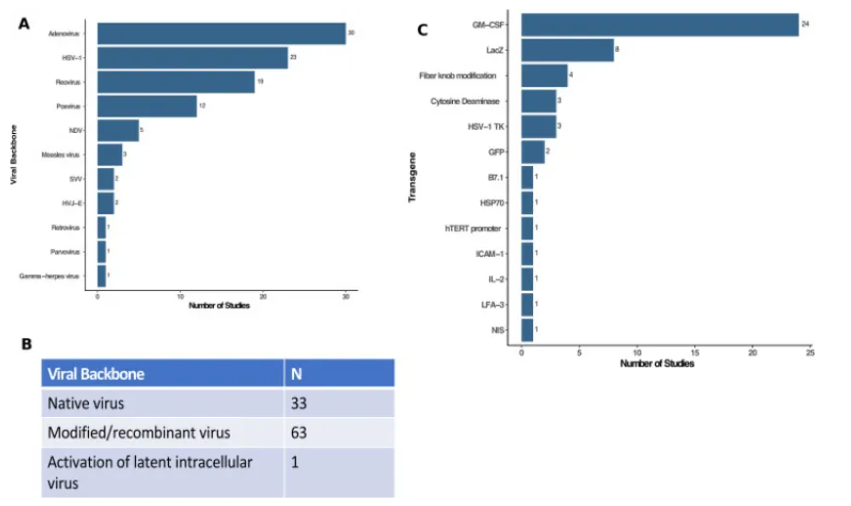
As depicted in the figure above, Adenoviruses and Poxviruses were the most commonly used viruses in OV therapy studies in 2020. While one-third of these studies utilized natural phenotype viruses, the remainder employed recombinant viruses.
Deleting non-essential viral genes emerges as the most common genetic manipulation in OV-focused clinical studies. This deletion prevents viruses from replicating in healthy cells and enables them to initiate a viral infection specific to tumor cells. Larger viruses can carry more eukaryotic transgenes, and deleting non-essential viral genes opens avenues for adding transgenes that encode molecules with more anti-cancer properties. The Lister strain of Vaccinia virus serves as an example of OV with deleted non-essential genes. This laboratory-generated virus is being tested against various cancers in animal models. Unlike the natural type of Vaccinia virus, the Lister strain lacks the thymidine kinase gene (TK). Deleting TK reduces the replication efficiency of the Vaccinia virus in healthy cells, while the typically heightened expression of the TK gene in cancer cells allows the virus to exert tumor-specific lytic effects without compromising replication. Besides deletions, adding transgenes can also enhance the anti-tumor effect of OVs. As seen in the figure above, at least 51 recombinant genes have been used or are being used in OV clinical studies. The Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) gene, known for promoting the development of local dendritic cells, is the most commonly used gene in OV vectors. It has been utilized in T-VEC to activate the host immune response in the tumor microenvironment.
Approved Therapies Based on HSV-1 Recombinant Virus
Before 2011, most therapies were ineffective for metastatic melanoma, except for IL-2 therapy, which showed limited efficacy in some cases. In 2011, BRAF and MEK inhibitors began to be effectively used against BRAF-mutant metastatic melanomas; however, this effect often proved short-lived due to the acquisition of resistance by metastatic melanoma.
Advancements in immunotherapies and the development of monoclonal immunotherapies targeting CTLA-4 and PD-1 proteins, such as ipilimumab and nivolumab, have somewhat overcome resistance to BRAF/MEK, but the side effects caused by these drugs in patients and their efficacy only in patients with these biomarkers limit their use. Generally, OVs have been observed to cause low levels of toxicity in clinical trials.
HSV-1 enters cells by targeting HVEM and nectin-1 proteins. HVEM expression is high in melanoma, and nectin-1 is a biomarker found in glioblastoma. T-VEC and Teserpaturev are HSV-1-based OV therapies approved for melanoma and glioblastoma, respectively, by various institutions.
Talimogene Laherparepvec (T-VEC)
is an FDA-approved drug based on a recombinant HSV-1 virus developed for metastatic melanoma treatment. Originally initiated under the name OncoVEXGM-CSF by the company BioveX, its patent transferred to AMGEN in 2011 upon BioveX’s acquisition by AMGEN, and the drug was renamed T-VEC. It received FDA approval in 2015 for the treatment of stage III and IV melanoma cases, thanks to successful results demonstrated in clinical trials.
HSV-1 is a large double-stranded DNA virus, and its extensive genome makes it amenable to genetic manipulation. The HSV-1 strain used in T-VEC, known as JS1, is an engineered strain with the ICP47 gene deleted. Deleting the ICP47 gene facilitates access of leukocytes’ MHC-I molecules to viral antigens and enhances the activation of antigen-specific immune responses.
In addition to the deletion of the ICP47 gene, the ICP34.5 gene encoding protein kinase R (PKR) inhibitor proteins is also removed. Protein kinase R is synthesized by many body cells and can degrade viral DNA. Deleting the PKR inhibitor gene in JS1 prevents the virus from replicating in healthy cells. Since cancer cells express low levels of PKR protein, the viral effect is not significantly weakened in these cells. Furthermore, to induce more immune responses and infiltration of T cells into the tumor microenvironment (TME), the GM-CSF gene, which activates dendritic cells, has been added to the viral genome.
In clinical trials, the efficacy of T-VEC treatment in stage III and IV melanoma patients was compared to GM-CSF treatment. The comparison revealed that patients treated with T-VEC had a significantly longer overall survival than those treated directly with GM-CSF (46.8 months vs. 21.5 months).
Teserpaturev
approved for the treatment of malignant glioma in Japan, has shown promising results in clinical trials for patients with prostate and malignant pleural mesothelioma, as well as recurrent olfactory neuroblastoma. Like T-VEC, Teserpaturev is a recombinant HSV-1 virus with deleted ICP34.5 and ICP47 genes; however, unlike T-VEC, it does not include the GSP-CSF gene, and the ICP6 gene is inactive.
The inactivation of the ICP6 gene disrupts the production of ribonucleotide reductase (RR), necessary for viral nucleotide synthesis. While this adversely affects the replication success of Teserpaturev in healthy cells, the modified virus can continue to replicate in tumor cells due to the disrupted DNA replication repair pathway and excessive nucleotide synthesis in these cells.
Other Approved OV-Based Therapies
H101
Oncorine/H101, produced in China, has been approved by the Chinese Ministry of Health for use in combination therapy for nasopharyngeal cancer. It is being tested for the treatment of head, neck, and esophageal squamous cell carcinomas. Tumor cell selection in H101 is achieved by deleting the EB1B-55kDa gene in the adenoviral vector. When used in combination with cisplatin therapy, H101 has been noted to exhibit tolerable side effects and significant antitumor effects.
The EB1B-55kDa gene is a potent suppressor of p53, and its deletion in the adenovirus allows the virus to replicate in cancer cells with p53 mutations while preventing replication in healthy cells with intact p53 production.
ECHO-7/Rigvir
ECHO-7 picornavirus has been approved for melanoma treatment in Latvia (2004), Georgia (2015), and Armenia (2016). Rigvir is the only non-recombinant OV therapy listed for approval; however, the manufacturer has been embroiled in scandals, and its success has been questioned by various authorities, leading to the cessation of production in 2019. The manufacturer’s scandalous involvement included exaggerating the amount of virus per dose and insufficient data to support initially positive results, leading to the end of production. Despite the controversies surrounding clinical data and outcomes, Rigvir has been shown to negatively impact the viability of melanoma, gastric adenocarcinoma, and other cancer cells in various in vitro studies.
Despite the scandals involving the manufacturer, in vitro studies suggest that Rigvir still possesses oncolytic potential and could potentially be reintroduced for clinical testing with advanced genetic manipulation strategies, similar to other approved OV therapies.
Optimization of OV Use in Cancer Treatment: Combination Therapies Most preclinical and clinical studies have demonstrated that OVs yield better results when used in conjunction with other conventional cancer treatments. The low side effect and toxicity profile of OVs ensures tolerability even when used in combination therapies without overlapping side effects. This is because OVs have unique anti-tumor mechanisms that differ from other therapies and have a low likelihood of synergy with the side effects of other treatments.
For example, immune checkpoint inhibitors (ICIs) targeting PD1 and PDL1 proteins have shown successful results when used in combination with OVs. ICIs are generally dependent on PD1-PDL1 expression and have limited efficacy against “cold tumors,” where immune cell infiltration is difficult. OVs can increase PD1 and PDL1 expression by inducing cellular IFNy release, synergizing with ICIs and enhancing treatment success against “cold tumors.” Various OVs, including T-VEC, have demonstrated successful effects in preclinical studies when used in combination therapy with ICIs.
Additionally, OVs have shown successful synergy with CAR-T cell therapies and TIL therapies. The inflammation induced by OVs leads to the release of cytokines such as TNF and IL-2 and chemokines such as CXCL9 & CXCL10, enhancing the immune response of T cells against tumors. Preclinical studies have also demonstrated that OVs may have a stronger effect when used in conjunction with chemotherapies and radiotherapies.
Clinical Challenges in the Development of OVs Despite the potential of OVs for cancer therapy, there are still significant challenges hindering their full potential utilization. Some of these challenges include the low tropism of OVs and the inability of animal models to fully reflect their effects in humans.
Compared to the HSV-1 virus, Vaccinia Virus is a more widely used OV type with high tropism, but its genome is shorter and less amenable to manipulation than HSV-1. The reason for working with HSV-1 in mice is because most mouse strains are resistant to HSV-1. Other clinical limitations in tropism and OV selection are significant barriers to the development of OV therapies.
OVs are generally administered directly into tumors through injection, and their intravenous administration may lead to neutralization by antiviral antibodies before reaching the tumor, limiting their biodistribution.
Drug delivery strategies such as biochemical coatings may provide protection to OVs until they reach the tumor, but this strategy can increase the cost of treatment development, production, and preservation.
The efficacy of OVs may also be limited in patients with pre-existing antiviral immunity. The optimization of OV delivery strategies and the impact of potential antiviral resistance in patients on treatment remain controversial and require further clinical studies.
Conclusion
In summary, OV therapy represents a unique cancer treatment method that utilizes animal viruses targeting tumors while showing minimal efficacy in healthy cells. In addition to selective viral lytic effects on tumors, they can activate anti-tumor immune responses by expressing eukaryotic anti-tumoral transgene loads in tumors. The tumor-specific infection and inflammation induced by OVs can disrupt the TMEs, rendering tumors more vulnerable to conventional cancer therapies such as other ICIs and immune cell therapies.
The limited number of OV cancer therapies approved by healthcare institutions since 2015 may indicate that this therapeutic approach is still in the developmental stage, despite the positive results obtained in numerous clinical trials. Further optimization of drug delivery strategies through more clinical studies and modeling is necessary for the widespread and successful use of OV cancer therapies. However, the preclinical and clinical success of this technique holds promise for the future.